Quality – Corrective and preventive action procedure

Corrective and preventive action procedure
What is a corrective and preventive action procedure?
A corrective and preventive action procedure is the process which a person or company takes when a specific task, activity, outcome or non-conformance has an issue which can be rectified or improved.
Corrective and preventive actions are a powerful quality tool, which can lead to continuous process improvements and timely rectifications - which improve project outcomes.
Different companies and different industries engage and partake in different corrective and preventive action procedures.
Corrective and preventive action procedures in construction or oil and gas may look quite different to the actual corrective and preventive actions taken in manufacturing or mining, but the end goal is the same.
The goal of a corrective and preventative action procedure is to:
- Eliminate detected conformities such as defects
- Eliminate the cause of a detected non-conformance or other undesirable situation
- proactively create preventive actions to eliminate the cause of potential non-conformances and undesirable situations
Together, these outcomes result in better procedures and better outcomes for projects and the business.
Why is a good corrective and preventive action procedure important?
Having a good and reliable corrective and preventive action procedure is critical to the success of your company. Without maintaining a strong procedure, you want catch and correct a number of non-conformances, which over time combine to reduce the quality of work.
Without a good procedure in place, workers spend and waste their time communicating non-conformances to the wrong people and losing or misplacing important and informative information.
When you have a good corrective and preventive action procedure in place, people understand the exact steps which need to be taken when a defect or process issue arises.
They know what documents or forms need to be completed, who needs to be contacted, and the exact procedure which will be followed.
This level of clarity (which can also be guaranteed and streamlined through corrective and preventive action software) improves the efficiency of your workers as well as your company.
What does a corrective and preventive action procedure look like?
As previously mentioned, the corrective and preventive action procedure required for your company an industry will differ from other companies and industries, but there are some fundamental steps in any good CAPA procedure which every company will take.
Step 1 - The first step in the corrective and preventive action procedure is to identify a required action. This identification could can occur when a non-conformance occurs (and requires immediate corrective action), or when an employee or workers spots a process or similar issues which could be improved by implementing a preventive action.
Step 2 - Acknowledgement and consultation - The next step, depending on the urgency and severity of the issue, is for someone or a group of people to acknowledge the issue, and then for a group of stakeholders to consult on the best path forward. This consultation is best when different levels of worker are involved - such as a site worker, quality manager and project manager - but sometimes minor issues can be solved with a smaller group.
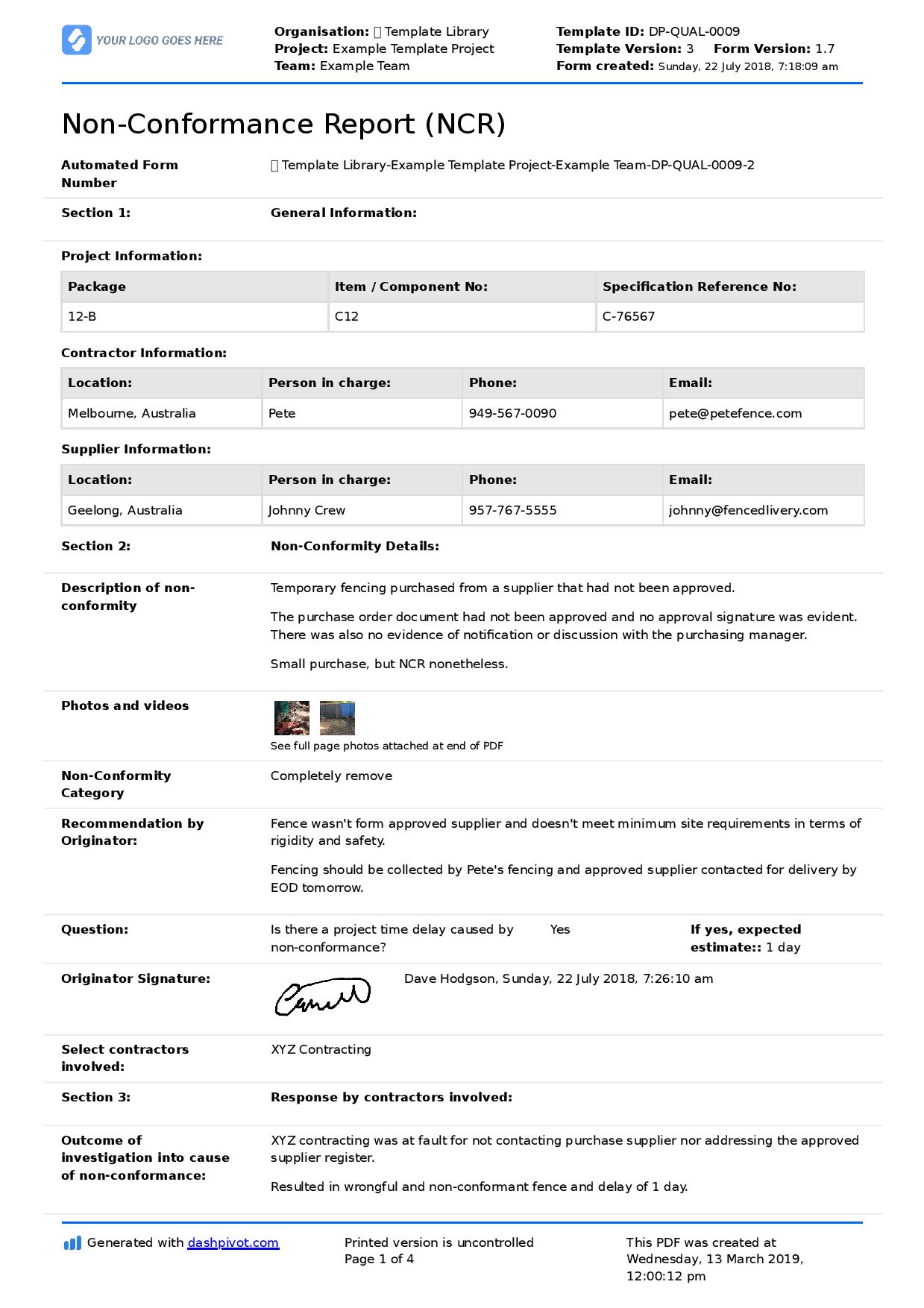
Step 3 - Recording - The next step (which can sometimes be the first step within smaller teams) is for the non-conformance or issue which surfaced the need for the corrective or preventive action to be recorded.
The mechanics behind how they are recorded can vary. Some companies include the actions - both corrective and preventive - within their non conformance report, while others use a single form for the actions.
I like when the corrective and preventive actions are recorded alongside the non conformance or issue, because it connects the two forever. It's easy for people to look back and see why the change was implemented, and then measure the results of that change over the initial issue.
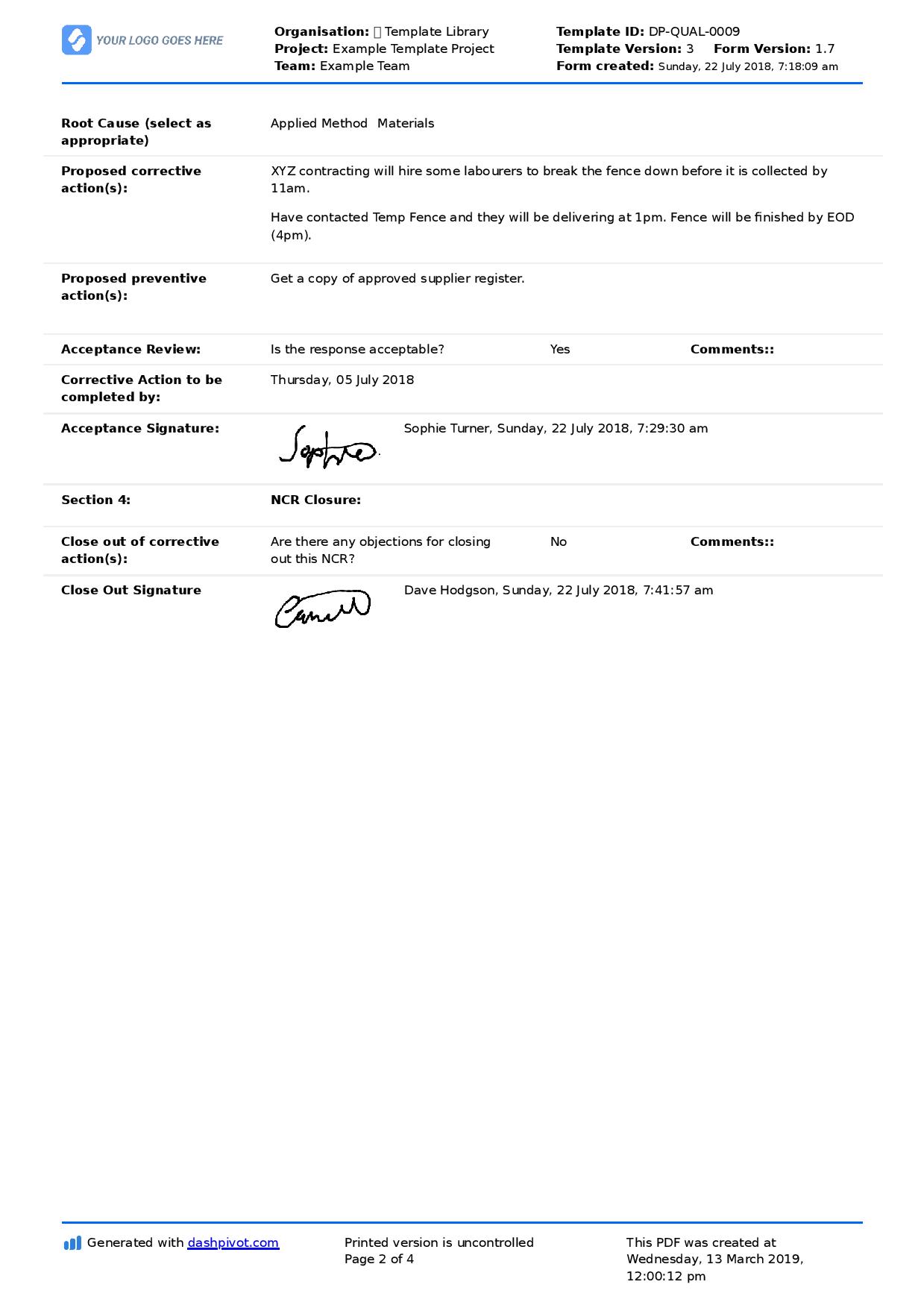
You can see an example corrective and preventive action report below, which outlines the NC and then proposes both a corrective and preventive action.
The last piece of the recording puzzle is that usually, companies will maintain a corrective and preventive action or NCR register which summarises and highlights all of the NCRs or CAPAs which the company has implemented. This is the source of truth when it comes to maintaining good records of these issues.
Use this corrective and preventive action procedure sheet for free.
Step 4 - Investigation - Investigations are not always required when a corrective or preventive action is recorded. But for major or highly influential issues, non-conformances or defects, an investigation may be launched.
Step 5 - Implementation - At this point, the team have decided that a preventive or corrective action needs to be implemented, have decided what one or each of those actions is, and recorded the proposed implementation.
The next stage is obviously implementation. This involves the responsible actioner or action person to implement the change. If it's a wide-reaching change, more than one person may be engaged, while other issues will require just one responsible person.
Step 6 - Action review and validation - The whole point of this corrective and preventive action procedure is to improve an issue or problem so that it doesn't happen again. So measuring the impact of your change is absolutely vital.
Your corrective or preventive action should have a positive impact on the process change or non-conformance, and this is what needs to be reviewed and validated for future reference and to make sure that processes are not being made worse (which can happen).
Avoiding corrective and preventive actions all together
A good corrective and preventive action procedure is only required for companies who make mistakes or can improve i.e those which are not perfect.
Unfortunately, this is all companies. But fortunately, companies can reduce the number of defects and non-conformance which happen on their projects.
This can be done by improving other processes and procedures alongside the corrective and preventive procedures in place - or companies can quickly upgrade their procedures through tools.
There are some great quality softwares and tools which help to streamline quality processes and procedures including defect management and inspections.
These tools can also automate and standardise procedures like your corrective and preventive action procedure.
Instead of having to manually send your NCR form to the applicable manager, when an NCR or issue is recorded, the manager or person is automatically notified so that the issue can be quickly consulted on, managed and rectified.
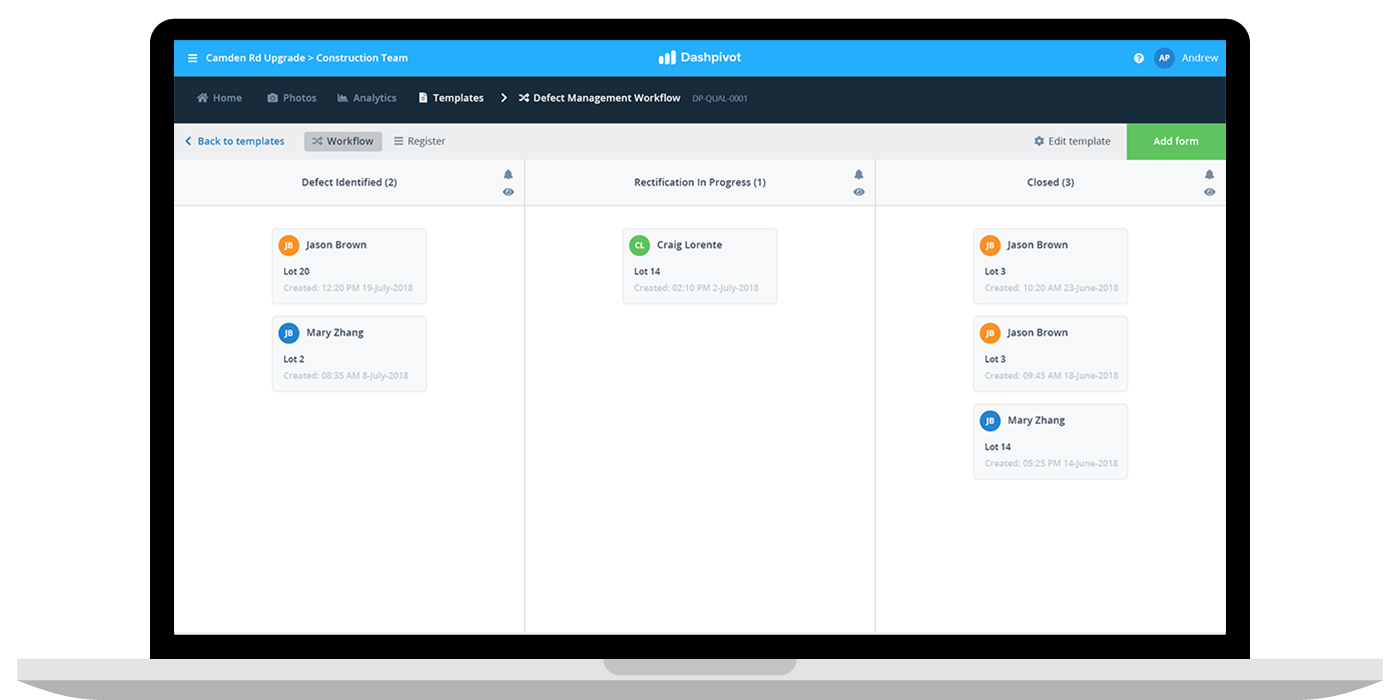
There are a bunch of improvements which most companies can make to their corrective and preventive procedure, some of which include technology and others which are more process and people driven.
Quality is a constant battle and endeavour for all companies - across many project, product and service based companies including construction, oil and gas, mining and manufacturing - and these companies also work interdependently.
A quality improvement by one company has a positive impact on other companies on the same project, with each company then having to fight less defects and delays, and projects moving forward more smoothly.
Even though in general, quality, quality control and quality outcomes continues to improve, corrective and preventive action procedures are going to be in play for a long time still.
People in 80+ countries use this quality management system to improve their quality processes and outcomes.